Great science. Innovative thinking. More progress, faster.
Creating solutions when traditional methods fail.
This typically includes the following steps:
- Analysis of the physical system to determine what biorelevant measurables are needed and model processes that may occur
- Experimental design to obtain the metrics
- Data analysis and interpretation to derive results that can be utilized in formulation science and/or regulatory interactions
Complex dosage form evaluation
- Design, bioequivalence, QA
- Ophthalmic products
- Physiologically relevant in vitro release testing (dissolution/release)
- Drug distribution
- Complex injectables—drug-protein complexes
- Drug release and distribution
- Manufacturing optimization
- Develop QA tests for ophthalmics and other complex dosage forms
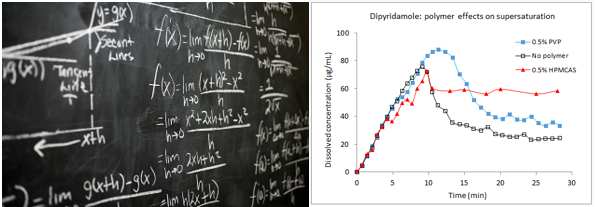
supersaturating formulations
- Supersaturating oral dosage forms to measure the absorbable dissolved concentration profile
- Screen for excipients that provide optimal bioavailability – can be done in any medium to predict performance in the GI fluids
- Applications include:
- Polymer screening for amorphous solid dispersions
- Nanoparticle delivery systems
- Neat amorphous API
- Lipid systems
Problem analysis
- Complex formulation physical modeling and analysis
- Ophthalmic products
- Drug-protein injectables
- Amorphous supersaturating formulations
- Data generation for product development & regulatory evaluation
- Dosage form in vitro and biopharmaceutical analysis to identify critical parameters
- Experimental method development
Biorelevant In vitro dissolution/release tests
- Customized release test development
- Ophthalmic, intranasal, and injectable formulations
- Supersaturated media precipitation/crystallization rates
- Release from semi-solid formulations
Dosage design for poorly soluble drugs
- Determine target absorption from pharmacokinetic data
- Design target in vitro dissolution to optimize bioavailability
- Solubilization formulation strategies and testing
- Drug-protein/polymer binding and release kinetics
Alliances and Business opportunities
- Ophthalmic and nasal products in the pipeline- ANDAs and NDAs
- Developing manufacturing technologies for amorphous solid dispersions (patents pending)
- Marketing partners
- Work with pharma companies developing ANDAs and NDAs
